|
|
I. Introduction
In this Vaccine Module, we presents how G-protein-coupled receptor (GPCR) could potentially play a role in the development of new vaccines, as shown in studies on our organism of interest, Rhipicephalus microplus, formerly known as Boophilus microplus. With its associated agricultural problems, this parasitic tick has a significant economic impact on a variety of livestock species.
| |
 |
Multiple-Choice Question: The purpose of this module is to
(A) introduce the organism of interest and its associated agricultural problems.
(B) investigate vaccine development.
(C) show how GPCRs play a role in the development of new vaccines.
(D) A and C only
(E) All of the above |
|
II. Rhipicephalus microplus
The common name for this species is either the “cattle ticks” or “southern cattle ticks.” They are small arachnids of the order Ixodida in the same subclass as mites. Ticks are ectoparasites (i.e., external parasites) living by hematophagy, feeding on the blood of mammals, and are known vectors of a number of diseases including Lyme Disease, Q Fever, Rocky Mountain Spotted Fever, tularemia, and bovine anaplasmosis. A vector is any agent (person, animal, or microorganism) that carries and transmits an infectious pathogen into another living organism [1,2,3,5,17].
| The scientific classification of the cattle tick [17]: |
Kingdom: |
Animalia |
| |
Phylum: |
Arthropoda |
| |
Class: |
Arachnida |
| |
Order: |
Ixodida |
| |
Family: |
Ixodidae |
| |
Genus: |
Rhipicephalus |
| |
Subgenus: |
Boophilus |
| |
Species: |
R. microplus |
The cattle tick has been found on organisms ranging from cattle to buffalo, horses, donkeys, goats, sheep, deer, pigs, dogs, and some other wild animals, most commonly in Asia, parts of Australia, Madagascar, Southeastern Africa, the Caribbean, South and Central America, and Mexico. Although the tick has been eradicated in the US [2,3,17], there are still sporadic occurrences in the buffer zone along the US-Mexican border. Cattle fever ticks have expanded beyond the quarantine zone in South Texas. The problem is that there are currently no vaccines approved for use within the US for controlling these vectors.
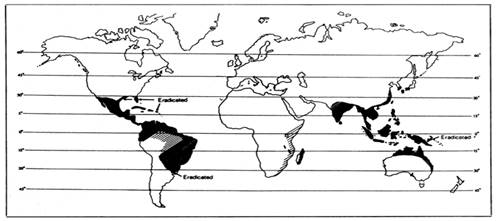
Figure 1: World-wide distribution of the Cattle Tick, Rhipicephalus microplus
R. microplus is considered to be the most important tick parasite of livestocks worldwide. Burdens of heavy tick infestations on animals can decrease production and damage cattle hides. R. microplus can also transmit protozoal parasites Babesia bigeminaand and Babesia bovis, causing babesiosis, and Anaplasma marginale leading to anaplasmosis [2,3].

Figure 2: The Cattle Tick, Rhipicephalus microplus
It is pertinent to study the life cycle of this tick in order to understand the mechanism of parasitism. R. microplus is a one-host tick with all stages spent on one animal. After hatching of the eggs, the larvae crawl up grasses or other plants to find their potential hosts, but they may also be blown by the wind. In the summer, the tick can survive for as long as 3 to 4 months without feeding. In cooler temperatures, they may live without food for up to 6 months. Ticks that do not find their host will eventually die of starvation. Newly attached seed ticks (i.e., larvae) are usually found on the softer skin inside the thigh, flanks, and forelegs; they may also be seen on the abdomen. After feeding, the larvae molt twice and become nymphs before turning into adults. The feeding takes place only once in each developmental stage (larva, nymph and adult) but over several days [17]. Adult male ticks become sexually mature after feeding, and mate with adult feeding females. A mated female tick detaches from the host and deposits a single batch of many eggs in the surrounding. These eggs are typically placed in crevices, debris, or under stones. The female tick dies after ovipositing. Ticks in the subgenus Boophilus have a life cycle that can be completed in 3 to 4 weeks; this characteristic can result in a heavy tick burden on animals [2,3,17]. Figure 3 below illustrates this cycle
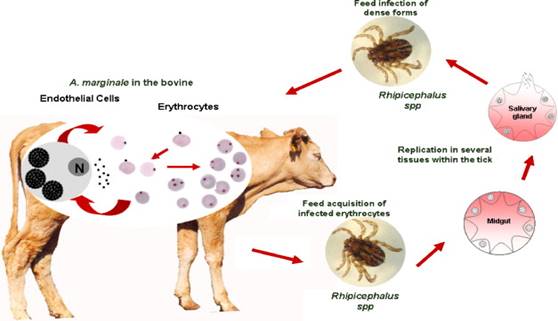
Figure 3: Life cycle of the Cattle Tick, Rhipicephalus microplus
In arthropod research, there is an absence of a complete Chelicerate genome, which includes ticks, mites, spiders, scorpions and crustaceans. Model arthropod genomes such as Drosophila and Anopheles are too taxonomically distant for a reference in tick genomic sequence analysis. The genome of R. microplus has an estimated size of 7.1 Gb, a very small one comparing to the human genome of over 3 billion bp. NCBI has a published Genome Assembly/Annotation report that can be found directly at http://www.ncbi.nlm.nih.gov/genome/?term=rhipicephalus%20microplus.
| |
 |
Fill in the Blanks and Multiple-Choice Questions:
- R. microplus is a type of _______ that parasitizes a variety of __________ species.
- In this relationship, the tick can be thought of as a _______ because it is an organism that carries and transmits disease.
- The life cycle of the tick can best be described by
| (A) |
Larvae molt twice to become nymphs and then adults. |
| (B) |
There are three stages of the life cycle: larva, nymph and adult. |
| (C) |
First eggs hatch and find a host, then larvae feed on their host, molt twice, and as adults they mate, detach from their host and die. |
| (D) |
Rhipicephalus microplus is a one-host tick. |
- During the tick life cycle, when is the host infected with the parasite?
| (A) |
During each developmental stage, the tick feeds once. Thus, all feedings are probable times to transmit the parasite to the host. |
| (B) |
Only adult ticks will transmit the parasite when feeding. |
| (C) |
Only the nymph stage ticks transmit the parasite. |
| (D) |
Only the larvae will transmit the parasite. |
|
III. Vaccines
To understand the goal of vaccine research, we need to know vaccine development and its history; vaccines are common biological preparations developed to improve immunity against particular diseases. A vaccine is typically made with a weakened or inactivated form of the disease-causing microbe, its surface proteins, or toxins. The goal of vaccination is to stimulate the immune system so that the body can recognize the vaccine as a foreign agent and develop an ability to neutralize the actual live microbe in future encounters [9,14,16].
Vaccines do not guarantee complete protection from a certain disease. For example, the host's immune system may not respond adequately or its immunity could have been lowered due to the lack of B-cells capable of constructing an antibody for that particular antigen [14]. Even if the host develops antibodies, its immune system may not be perfect. If re-infected, the immune system may not be able to defeat the foreign agent immediately, and in this case, however, the infection would be less severe than normal and would heal faster [9].
A. Vaccine Performance
Vaccine Efficacy is defined as the reduction in the incidence of a disease by comparing vaccinated and unvaccinated people. The is usually measured during phase I or II of clinical trials by giving one group a vaccine and comparing the incidence of disease in that group to another group of people who did not receive the vaccine. Ideally, these would be double-blind, randomized, and controlled trials [15,18,19,20]. The equation is
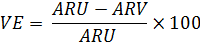
| |
where |
VE |
= |
vaccine efficacy |
| |
|
ARU |
= |
attack rate in the unvaccinated population |
| |
|
ARV |
= |
attack rate in the vaccinated population |
Advantages: A vaccine efficacy study includes rigorous control for biases afforded by randomization, prospective, active monitoring for disease attack rates, and careful tracking of vaccination status; often there is, at least for a subset of the study population, laboratory confirmation of the infectious outcome of interest and a sampling of vaccine immunogenicity.
Disadvantages: The complexity and expense of performing them, especially for relatively uncommon infectious outcomes for which the sample size required is driven upwards to achieve clinically useful statistical power.
Ultimately, vaccine efficacy studies measure outcomes beyond disease attack rates (i.e., proportion of subjects who become sick from exposure to the vaccine or not), including hospitalizations, medical visits, and costs. The external validity of the results of a vaccine efficacy study to a larger, non-study population may be lowered by differences between the study cohort and the population as a whole [15].
Vaccine Effectiveness is, often confused with vaccine efficacy, defined as a “real world” view of how a vaccine reduces disease in a population, despite having a high vaccine efficacy in prior well-controlled clinical trials. This measure can assess the net balance of benefits and adverse effects of a vaccination program, not just the vaccine itself, under more natural field conditions rather than those controlled conditions in a clinical trial. Vaccine effectiveness is proportional to vaccine potency (i.e., vaccine efficacy) but is also affected by how well target groups in the population are immunized [4,10]. The outcome data (i.e., vaccine effectiveness) are expressed as a rate difference, with use of the odds ratio (OR) for developing infection despite vaccination: Effectiveness = (1 – OR) x 100.
B. Vaccine Efficacy Comparisons Based on the Type of Study
Method of Study |
Advantages |
|
Disadvantages |
Screening |
Rapid and uses few resources, inexpensive [9,15]. |
|
Inaccuracy based on the proportion of the population vaccinated and cases that are vaccinated [9,15]. |
Outbreak Investigations
- Total Census
- Clusters
|
-
Very common;
-
Accurate because it allows for the collection of cases;
-
When AR is high, the exposure of vaccines and non-vaccines to disease become comparable;
-
Very easy to perform [15,19].
|
|
-
Requires more resources than screening;
-
Exposure of vaccines and exposure of non-vaccines to disease may not be absolutely equivalent [15,19].
|
Secondary Attack Rates
- Families
- Clusters
|
-
Corrects for potential differences in exposure between vaccinated and unvaccinated;
-
Potentially, one can add results from many family investigations together, allowing for a more accurate estimate;
-
Assuming exposure within clusters is comparable from one cluster to another, results of multiple clusters can be combine [15,19].
|
|
-
Probably requires more resources than an outbreak investigation;
-
Only a small number of children will be in the proper age group in a given family, so must visit many families;
-
Case definition less predictive in the absence of an outbreak;
-
Needs further evaluation of uniformity of exposure within clusters [15,19].
|
Endemic Areas |
-
Requires only minor changes of a particular technique;
-
Requires similar resources as a coverage survey;
-
Minor changes to design will provide coverage data simultaneously [15,16].
|
|
-
Relies on the parent’s diagnosis and recall of the disease rather than clinical information;
-
Coverage assessments for a specific group, may increase the number of individuals per cluster [15,16].
|
Case-Control Studies |
Allows maximal resources to be utilized in finding the vaccination status of cases and a few matched controls instead of the entire population [15,19]. |
|
Gives a falsely high vaccine efficacy if the AR in the vaccinated are high [15,19]. |
Cohort Studies |
-
Clearly demonstrate an appropriate temporal sequence between exposure and outcome;
-
Permits the direct calculation of incidence rates of both vaccinated and unvaccinated;
-
These studies permit multiple outcomes to be assessed in the same study;
-
Can be used for relatively uncommon diseases [19].
|
|
-
Potentially large sample size requirements;
-
Very long follow-up periods;
-
A need to reassess exposure status on a frequent basis;
-
Due to the relatively low incidence and long latency periods for certain diseases, sometimes thousands of subjects must be enrolled and followed for years/decades in order to obtain enough data for valid statistical comparisons [19].
|
Table 1: Vaccine efficacy comparison table based on particular methods of study
C. Vaccine Efficacy Study Vocabulary
-
Screening is a strategy used in a population to identify an unrecognized disease in individuals without signs or symptoms. This can include individuals with pre-symptomatic or unrecognized symptomatic disease. As such, screening tests are somewhat unique in that they are performed on persons apparently in good health [9].
-
Outbreak is a term used in epidemiology to describe an occurrence of disease greater than would otherwise be expected at a particular time and place. It may affect a small and localized group or impact upon thousands of people across an entire continent. Two linked cases of a rare infectious disease may be sufficient to constitute an outbreak. Outbreaks may also refer to epidemics, which affect a region in a country or a group of countries, or pandemics, which describe global disease outbreaks [9].
-
Attack Rate (AR) is the biostatistical measure of frequency of morbidity, or speed of spread, in an at risk population. An at-risk population is defined as one that has no immunity to the attacking pathogen which can be either a novel or established pathogen. It is used to project the number of victims to expect during an epidemic. This aids in marshalling resources for delivery of medical care as well as production of vaccines and/or anti-viral and anti-bacterial medicines [15,19].
-
Secondary Attack Rate is defined as the probability that infection occurs among susceptible persons within a reasonable incubation period following known contact with an infectious person or an infectious source. It is a key epidemiologic parameter in infectious diseases that are transmitted by contact. It can be estimated using a variety of epidemiologic study designs, models, and methods of estimation. Inference needs to take into account the correlation of susceptibles exposed to the same infectious source [15,19].
-
Endemic is a condition of an infectious disease when the infection is maintained in the population without the need for external inputs of new cases. For example, chickenpox is endemic in the UK, but malaria is not [16].
-
Pandemic is an epidemic of infectious disease that has spread through human populations across a large region; for instance multiple continents, or even worldwide. A widespread endemic disease that is stable in terms of how many people are getting sick from it is not a pandemic. Further, flu pandemics generally exclude recurrences of seasonal flu. Throughout history there have been a number of pandemics, such as smallpox and tuberculosis. More recent pandemics include the HIV pandemic as well as the 1918 and 2009 H1N1pandemics [16].
-
Cohort Study is a research method to investigate a group of patients and follow them over time. An example is the Nurses' Health Study, in which over 20,000 nurses were identified and followed up annually with tests and surveys for over 25 year. These studies provide very valuable information, but are obviously very expensive and time-consuming. In most cohort studies, one wants to assemble patients without the disease in question, and then follow them until they develop the disease. By comparing the characteristics of patients with and without disease, one can identify risk factors [19].
-
Case Control Study is a type of studies in which patients who already have a specific condition are compared with people who do not. They often rely on medical records and patient recalls for data collection. This type of studies isoften less reliable than randomized controlled trials and cohort studies because showing a statistical relationship does not mean than one factor necessarily caused the other [19].
D. Types of VaccinesThe most common types of vaccinations represent different strategies used to reduce the risk of illness or infection, while having the ability to induce an immune response [9,16].
-
Killed: The microbes used in this type of vaccination are not living or reproducing, they were previously virulent, but have been destroyed through chemicals, heat, radioactivity, or antibiotics.
-
Attenuated: These vaccines contain live, attenuated organisms. Many times the organism has been cultivated under conditions which disable their virulent properties (i.e., weakened the organism). Most of these vaccines are for viruses, however, there are some exceptions for bacteria.
-
Toxoid: Made from the toxin the organism produces, rather than the organism itself.
-
Subunit: This refers to a vaccine that uses fragments of the organism to induce an immune response, for example, only using surface proteins.
E. The Cattle Tick Vaccine
Babesiosis or “cattle fever” was eradicated from the United States, from 1906 to 1943, by eliminating its vectors R. microplus and Rhipicephalus annulatus (a similar tick species). Before its eradication, babesiosis costed the US an estimated $130.5 million in direct and indirect annual losses; in current dollars, the equivalent would be $3 billion [5]. R. microplus and R. annulatus still exist in Mexico, and a permanent quarantine zone is maintained along the US-Mexican border to prevent their reintroduction into the US. Within this zone, the USDA’s Animal and Plant Health Inspection Service (APHIS) conducts a surveillance program to identify and treat animals infested with these exotic ticks. Recently, increased numbers of infestations have been recorded in the quarantine zone [5]
Figure 4: Heavy infestation of cattle ticks
Control of cattle tick infestations has been primarily by application of acaricides, which has resulted in environmental pollution and the unwanted selection of resistant ticks. Arcaricides are pesticides used to kill ticks and mites [5], used commonly in agriculture with very high toxicity. “Commercial tick vaccines for cattle based on the R. microplus Bm86 gut antigen have proven to be a feasible tick control method that offers a cost-effective, environmentally friendly alternative to the use of acaricides" [1]. The current problem is that cattle fever ticks have expanded beyond the quarantine zone in southern Texas. While Bm86 vaccine has been developed in Australia and Cuba, there are no vaccines approved for use within the US for controlling these vectors.
The Bm86 vaccine is a glycoprotein, located
predominantly on the surface of tick mid-gut digestion cells, without known functions [1,5]. As a sidenote, glycoproteins are proteins that contain oligosaccharide chains (glycans) covalently attached to polypeptide side-chains. The carbohydrate is attached to the protein in a co- or post-translational modification. Glycoproteins are often important integral membrane proteins, where they play a role in cell–cell interactions. Glycoproteins are also formed in the cytosol, but their functions and the pathways producing these modifications in this compartment are less well-understood [6]. Bm86 is a recombinant antigen identified through a complex series of protein fractionations followed by vaccination trials in cattle to assess the antigenic efficacy against R. microplus [1].
The Bm86 vaccine has variable efficacy against cattle fever ticks. A possible explanation for this variation in vaccine efficacy is amino acid sequence divergence between the recombinant Bm86 vaccine component and native Bm86 expressed in ticks from different geographical regions. Commercially available vaccines against cattle fever ticks, which are approved for use outside of the US, including Gavac® (Heber Biotec; Havana, Cuba), TickGARD (Hoechst Animal Health; Australia), and TickGARDPLUS (Intervet Australia; Australia), are based on the recombinant form of the concealed midgut antigen, Bm86 [1,5].
Figure 5: Commercially available vaccines against cattle fever ticks
Commercial tick vaccines reduced tick infestations on cattle and the intensity of acaricide usage, as well as increasing animal production and reducing transmission of some tick-borne pathogens. Commercialization of tick vaccines has been difficult owing to previous constraints of antigen discovery, the expense of testing vaccines in cattle, and company restructuring, the success of these vaccines over the past decade has clearly demonstrated their potential as an improved method of tick control for cattle [1]. Development of improved vaccines in the future will be greatly enhanced by new and efficient molecular technologies for antigen discovery and the urgent need for a tick control method to reduce or replace the use of acaricides, especially in regions where extensive tick resistance has occurred.
F. Vaccines and GPCR
GPCR is one of the most commonly used and successful targets for drugs with approximately 40-50% of all modern medicines interact with this protein group. GPCRs are prime targets of interest due to their physiological relevance. GPCR’s role as contributor to the information flow into cells also makes that they are associated with a multitude of diseases. The prominent role of GPCR in drug development will most likely increase in the future [8].
| |
 |
Fill in the Blanks:
- ____________ are common biological preparations that have been developed to improve ____________ for particular diseases.
- Some of the most common types of vaccines are ____________, which contain the non-living species being targeted, and ____________, which contain live organisms.
- One of the most commonly used and successful targets for drugs is a specific protein group called ____________.
|
IV. G-Protein Coupled Receptors (GPCRs)
GPCR is also known as 7-transmembrane protein (7TM) receptors, heptahelical receptors, serpentine receptors, or G-protein-linked receptors, which is a very large family of protein receptors. The main function of GPCRs is to sense stimuli from outside of the cell and activate signal transduction pathways inside the cell and ultimately to produce the biological responses. GPCRs are considered transmembrane receptors because they pass entirely through the cell membrane. They are called 7-transmembrane receptors because the proteins pass through the cell membrane exactly 7 times [6,7,8,11,12,13].
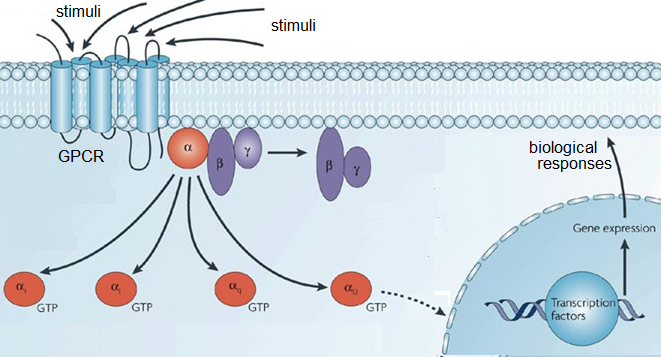
Figure 6: GPCR and intracellular responses to stimuli
Although GPCRs are the largest and most diverse group of membrane receptors, they are found only in eukaryotes, which are organisms with cells containing complex structures enclosed within membranes [6,12]. GPCRs are located on the cell surface, which is a prime location for receiving a variety of signals in the form of light energy, peptides, lipids, sugars, neurotransmitters, or proteins (the red dot in Figure 7). GPCRs have a wide variety of functions not limited to stimulus-response pathways and have also been linked to a wide array of biological and pathological conditions; they are thus very common drug targets [7,8,12]. Current research challenges include understanding GPCR regulation related to both normal and abnormal cellular processes.
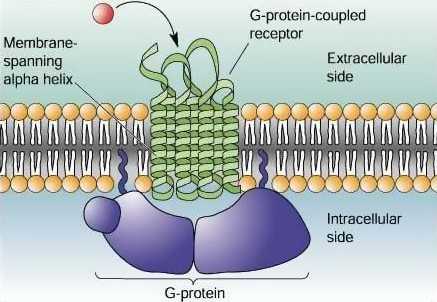
Figure 7: The 7 transmembrane proteins of GPCR and the stimulus (red dot)
A. GPCR Vocabulary
-
G-Proteins (guanosine nucleotide-binding proteins): A family of proteins involved in transmitting signals from a variety of different stimuli outside the cell into the inside of the cell. G-proteins function as molecular switches, meaning, their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When G-proteins bind GTP, they are activated (“turned on”), and when they bind GDP they are deactivated (“turned off”). G-proteins located within the cell are activated by GPCRs [6].
-
Receptor: It is a molecule, usually found on the cell surface, that receives chemical signals from outside the cell (see Figure 6). When external signals bind to a receptor, this action directs the cell to do something [8].
-
Transmembrane: It is a characteristic of a protein that spans across the entire cellular membrane. Transmembrane proteins typically function as gateways to allow or deny the transport of particular substances across the biological membrane. As a response to binding a signal, transmembrane proteins may have special ways of conformational changes (folding or bending) that allows the substance to transport across the membrane barrier [11].
-
Signal Transduction Pathways: A cellular process occurs when an extracellular signaling molecule (outside the cell) activates a cell surface receptor. In turn, the receptor alters intracellular molecules (inside the cell), creating a specific cellular response. The steps in signal transduction include: a signaling molecule activates a specific receptor protein on the cell membrane, or a second messenger transmits the signal into the cell, this causes specific physiological response [11]. In either step, the signal can be amplified.
-
Eukaryotes: It is a type of organisms with cells containing complex structures enclosed within membranes. The defining membrane-bound characteristic differentiating eukaryotes from prokaryotes is the nucleus. It is important to note that GPCRs are found only in eukaryotes, including yeasts and choanoflagellates, in addition to all animals.
B. GPCR Structure GPCRs are integral membrane proteins and they possess 7 membrane spanning domains (as can be deduced from their name and seen in Figure 7 above). Most GPCRs share a similar architecture and structure (conserved through evolution). The GPCR itself consists of a single polypeptide folded into a globular shape, and embedded into a cellular membrane. There are segments of the polypeptide loop inside and outside of the cell. Also, extracellular loops form part of the pocket, where signaling molecules bind to GPCRs [7,11,13].
C. Physiological Roles of GPCRs
Initially, GPCRs interact with G-proteins in the plasma membrane; they are specialized proteins with the ability to bind the nucleotides guanosine triphosphate (GTP) and guanosine diphosphate (GDP) [13]. The G-proteins that interact with GPCRs are heterotrimeric, meaning the protein has three different subunits (alpha-, beta-, and gamma-subunit). Alpha- and gamma-subunits are attached to the plasma membrane of the cell through lipid anchors. Then, external signaling molecules bind to a GPCR, causing conformational changes, triggering the interaction between the GPCR and the G-protein [11].
The mechanisms, or steps of GPCR in relation to their physiological roles include first ligand binding, then conformational changes in the structure, activation or deactivation, signaling and regulation. Ligand binding is the first step in a signal transduction pathway, when signaling molecule activates a cell surface receptor. The receptor then undergoes conformational changes, involving disruption of a strong ionic interaction between the 3rd and 6th transmembrane helices. The next step is G-coupled protein activation or deactivation. The conformational changes typically facilitate activation of the G-protein heterotrimer. Depending on the type of G-protein to which the receptor is coupled, a wide variety of downstream signaling pathways can be activated (based on highly specific interactions). GPCR Signaling can be independent or dependent. Then the receptor is regulated [6,7,8,11,12,13].
D. Classification of GPCRs
GPCRs are a superfamily of proteins (how many exist is unknown). The superfamily is divided into 3 main classes (A, B, and C) plus 3 other classes (D, E, and F) that are rare and unique. Despite the lack of detectable sequence similarity among the classes, the structure and mechanisms of signal transduction are similar across all GPCRs. It is interesting that the human genome encodes thousands of GPCRs. Also, there are several web-servers and bioinformatics tools available to predict GPCR sites and their classifications based on their amino acid sequence [6,8].
GPCR Class A (Rhodopsin-like) receptors are the largest class of GPCR genes (accounts for nearly 85%). This group can be subdivided into 19 more specific groups. This is a widespread protein family which includes hormones, neurotransmitters, and light receptors. These molecules all allow for extracellular signaling through their interaction with G-proteins.
GPCR Class B (Secretin Receptor Family) receptors are typically regulated by peptide hormones from the glucagon hormone family. Three subfamilies have been recognized in this class.
GPCR Class C (Metabotropic Glutamate/Phermone) receptors are a type of glutamate receptor that is activated indirectly through certain metabotropic processes. Glutamate receptors always bind the amino acid glutamate. This amino acid functions as an excitatory neurotransmitter.
GPCR Class D (Fungal Mating Pheromone Receptors) are involved in the response to mating factors on the cell membrane. Two specific receptors are STE2 and STE3. The amino acid sequences for both receptors contain high amounts of hydrophobic residues grouped into seven domains (believed to be very similar to the structure of GPCRs).
GPCR Class E (Cylic AMP Receptors) usually occur in slime molds. These receptors coordinate the aggregation of individual cells into a multicellular organism. They also regulate the expression of developmental genes.
GPCR Class F (Frizzled/Smoothened) refers to a set of GPCRs serving as receptors in many signaling pathways. Frizzled proteins play a key role in determining cell polarity, embryonic development, neural synapses, cell proliferation, and many other processes. This differs from smoothened (SMO) because these proteins are the molecular target for teratogen cyclopamine encoded by the SMO gene.
E. GPCR Prediction Servers
There are several web-servers and bioinformatics tools available to predict GPCR sites and their classifications according to their amino acid sequences.
1. PRED GPCR http://athina.biol.uoa.gr/bioinformatics/PRED-GPCR/input.htm
This server adopts a probabilistic approach with a highly discriminative profile Hidden Markov Model (HMM) excised from low entropy regions of multiple sequence alignments (MSA) to derive potent family features. This server uses HMM library construction for GPCR prediction. Initially, MSA are constructed from a subset of the data. Then, MSA blocks of low entropy are selected and overlapping windows with predefined maximum width of 21 columns were created. For each window, an HMM was created with the HMMER software package. Performance of HMMs as family classifiers was estimated after a HMM search pass through the membrane receptors dataset. Only those HMMs with high selectivity and sensitivity for families were selected from the HMM library. There are also certain criteria for HMMs to join the library, which include the calculated value of an estimator of discriminatory performance. This may actually contribute to PRED GPCRs stringent nature. Also, the E-value of the first false positive hit, and the E-value of the last true positive hit contribute to joining the library.
2. GPCR HMM http://gpcrhmm.sbc.su.se/
This server is also based on HMM that mimics the common topology of GPCRs. First, a training set is compiled with a redundancy reduced training set of 311 GPCRs from 11 families. The HMM library construction is based on the observed length and amino acid composition features. The model is compartmentalized whereby each transmembrane helix and loop region can be modeled by a separate compartment with specific amino acid probability distribution and length model. Two types of architecture are used to model loop lengths. In the first, loops highly conserved in length (cytosolic loops 1 & 2) are modeled in a way that restricts length to a finite maximum value. The other option is that loops are allowed to be infinitely long with decreasing probability. The advantages of this server are that the method can be locally installed and the server allows for uploading multiple sequences at once.
3. GPCR-GIA http://peds.oxfordjournals.org/content/22/11/699.long
GPCR-GIA utilizes a 2-layer ensemble classifier and introduces a novel scale called grey-incident degree with Grey Incidence Analysis (GIA). The server takes into consideration the amino acid composition without losing sight of the sequence itself (called pseudo amino acid composition), which often happens when amino acid composition is a feature. In regard to constructing a training set, the authors were concerned because the data sets constructed to train on existing predictors cover very limited GPCR family classes. To construct a higher quality benchmark, the following actions were taken. First, those sequences included maintained clear annotations, while equences deemed "probable," "potential," etc. were ruled out. Sequences annotated as "fragments" were
excluded, and there was a reduction in homology bias. The unique feature of GIA is its utilization of K Nearest Neighbor Classifier. According to the KNN rule, the query protein should be assigned to the subset represented by a majority of its KNNs. Authors utilize a novel scale called the grey incidence degree to measure the ‘nearness’ for the KNN classifier.
4. GPCRpred http://www.imtech.res.in/raghava/gpcrpred/
The general method of this server supports vector machine based method developed for annotation GPCRs on the basis of dipeptide composition. This method involves three steps for recognizing GPCRs from protein sequences and further classifying GPCRs into a family and subfamily.
5. PCA-GPCR http://www1.spms.ntu.edu.sg/~chenxin/PCA_GPCR/index.html
The general method of this server uses a comprehensive set of 1497 sequence-derived features. Principal component analysis is employed to reduce the dimension of the feature space to 32. The 32-feature vectors are then handed to a classification algorithm called intimate sorting, which predicts GPCRs at 5 levels, corresponding to whether the protein is a GPCR and then all of the subsequent families and subfamilies.
| |
 |
Fill in the Blanks:
-
____________ are common biological preparations that have been developed to improve ____________ for particular diseases.
-
Some of the most common types of vaccines are ____________, which contain the non-living species being targeted, and ____________, which contain live organisms.
-
One of the most commonly used and successful targets for drugs is a specific protein group called ______________________________.
True or False:
-
Vaccines are common biological preparations that have been developed to improve immunity for particular diseases.
(A) True
(B) False
-
The difference between vaccine efficacy and vaccine effectiveness is that vaccine efficacy is defined as a “real world” view of how a vaccine reduces disease in a population and vaccine effectiveness is defined as the reduction in the incidence of a disease among people who have received the vaccine compared to the incidence in unvaccinated people.
(A) True
(B) False
-
One of the most commonly used and successful targets for drugs is a specific protein group called G-Protein Coupled Receptors (GPCRs).
(A) True
(B) False
|
V. Rhipicephalus microplus, Vaccinations, GPCR, and Their Relatedness
Rhipicephalus microplus is an economically important tick that parasitises a variety of livestock species. Control of cattle tick infestations has been primarily by application of acaricides, which has resulted in selection of resistant ticks and environmental pollution. In other countries, the success of vaccines over the past decade has clearly demonstrated their potential as an improved method of tick control for cattle. Currently, there are no vaccines approved for use within the United States for controlling R. microplus. Development of improved vaccines in the future will be greatly enhanced by new and efficient molecular technologies for antigen discovery and the urgent need for a tick control method to reduce or replace the use of acaricides, especially in regions where extensive tick resistance has occurred.
One of the most commonly used and successful targets for drugs is a specific protein group called G-Protein Coupled Receptors (GPCRs). Approximately forty to fifty percent of all modern medicines interact with this protein group. GPCRs are prime targets of interest due to their physiological relevance. GPCR’s role as contributor to the information flow into cells also makes that they are associated with a multitude of diseases. The prominent role of GPCR in drug development, will most likely increase in the future.
| |
 |
Fill in the Blanks:
-
The main function of GPCRs is the ability to sense molecules outside the ____________ and activate ____________ pathways inside the cell, ultimately, to create ____________.
-
A ____________ is a molecule, usually found on the surface of a cell, that receives chemical signals from outside the cell.
-
There are ____________ classes of GPCRs. These classes maintain similar architecture and their ____________ has been maintained through evolution. The largest class of GPCRs is class ____, also known as rhodopsin-like receptors.
|
| |
|
Word Bank for "Fill in the Blanks" Questions:
Tick
Vector
Vaccines
Immunity
Toxoid
A
Cell
B
|
Vaccine Effectiveness
Six
Receptor
Structure
Killed
Ten
Cellular Responses
|
Subunit
Livestock
Attenuated
GPCRs
Vaccine Efficacy
Signal Transduction
Function |
|
VI. References
[1] Canales, M., Almazan, C., Naranjo, V., Jongejan, F., and de la Fuente, J. (2009) Vaccination with recombinant Boophilus annulatus Bm86 ortholog protein, Ba86, protects cattle against B. annulatus and B. microplus infestations. BMC Biotechnology. 9:29. http://www.biomedcentral.com/1472-6750/9/29. Accessed September 4, 2014.
[2] AHP Disease Manual (2014) Cattle Tick. http://www.spc.int/lrd/ext/disease_manual_final/cattle_tick.html. Accessed September 4, 2014.
[3] Bellgard, M.I., Moolhuijzen, P.M., Guerrero, F.D., Schibeci, D., Rodriguez-Valle, M., Peterson, D.G., Dowdf, S.E., Barrero, R., Hunter, A., Miller, R.J., Lew-Tabor, A.E. (2012) CattleTickBase: An integrated Internet-based bioinformatics resource for Rhipicephalus (Boophilus) microplus. Internat. J. Parasitol. 42:161-169. http://www.sciencedirect.com/science/article/pii/ S0020751911002815. Accessed September 4, 2014.
[4] Perencevich, E. (2013) Controversies in Hospital Infection Prevention: How Should We Calculate Influenza Vaccine Effectiveness? http://haicontroversies.blogspot.com/2013/01/whats-up-with-cdc-influenza-vaccine.html. Accessed September 5, 2014.
[5] Rodriguez-Valle, M., Taoufik, A., Valdés, M., Montero, C., Ibrahin, H., Hassan, S.M., Jongejan F., and de la Fuente J. (2012) Efficacy of Rhipicephalus (Boophilus) microplus Bm86 against Hyalomma dromedarii and Amblyomma cajennense Tick Infestations in Camels and Cattle. Vaccine 30(2012)3453–3458. http://www.sciencedirect.com/science/article/pii/ S0264410X12003830. Accessed September 4, 2014.
[6] Dorsam, R.T. and Gutkind, J.S. (2007) G-protein-coupled receptors and cancer. Nature Reviews Cancer 7, 79–94. http://www.nature.com/nrc/journal/v7/n2/full/nrc2069.html. Accessed September 5, 2014.
[7] Venkatakrishnan, A.J., Deupi, X., Lebon, G., Tate, C.G., Gebhard F. Schertler, G.F., and Babu, M.M. (2013) Molecular signatures of G-protein-coupled receptors. Nature 494, 185–194. http://www.nature.com/nature/journal/v494/n7436/full/ nature11896.html. Accessed September 5, 2014.
[8] Bertile, H. G. (2009) Protein-coupled Receptor. Scholarpedia 4(12):8214. http://www.scholarpedia.org/article/G_protein- coupled_receptor. Accessed September 5, 2014.
[9] The College of Physicians of Philadelphia (2014) History of Vaccines. http://www.historyofvaccines.org. Accessed September 5, 2014.
[10] Kelly, H., Carville, K., Grant, K., Jacoby, P., Tran, T., and Barr, I. (2009) Estimation of Influenza Vaccine Effectiveness from Routine Surveillance Data. PLoS ONE 4(3): e5079. doi:10.1371/journal.pone.0005079. http://www.plosone.org/article/ info%3Adoi%2F10.1371%2Fjournal.pone.0005079. Accessed September 6, 2014.
[11] Kobilka, B.K. (2007) G Protein Coupled Receptor Structure and Activation. Biochim. Biophys, Acta 1768(4):794-807. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1876727. Accessed September 6, 2014.
[12] Kroeze, W.K., Sheffler, D.J., and Roth, B.L. (2003) G-Protein Coupled Receptors at a Glance. J. Cell Sci. http://jcs.biologists.org/content/116/24/4867.long. Accessed September 6, 2014.
[13] Lodish, H. et al. (2000) G-Protein Coupled Receptors and Their Effectors. In: Molecular Cell Biology, 4th Ed., Section 20.3. http://www.ncbi.nlm.nih.gov/books/NBK21718. Accessed September 6, 2014.
[14] National Vaccine Information Center - Your Health. Your Family. Your Choice. http://www.nvic.org/. Accessed September 6, 2014.
[15] Orenstein, W.A., Bernier, R.H., Dondero, T.J., Hinman, A.R., Marks, J.S., Bart, K.J., and Sirotkin, B. (1985) Field Evaluation of Vaccine Efficacy. Bulletin of the World Health Organization 63(6):1055-1068. http://www.ncbi.nlm.nih.gov/pmc/articles/ PMC2536484. Accessed September 6, 2014.
[16] Centers for Disease Control and Prevention (2012) Principles of Epidemiology in Public Health Practice. http://www.cdc.gov/ ophss/csels/dsepd/SS1978/SS1978.pdf. Accessed September 6, 2014.
[17] Shapiro, L. (2013) Rhipicephalus Microplus - Southern Cattle Tick. Encyclopedia of Life. http://eol.org/pages/515050/ overview. Accessed September 6, 2014.
[18] Smith, D.J. (2003) Applications of Bioinformatics and Computational Biology to Influenza Surveillance and Vaccine Strain Selection. Vaccine 21(16):1758–1761. http://www.ncbi.nlm.nih.gov/pubmed/12686090. Accessed September 6, 2014.
[19] Smith, P.G., Rodrigues, L.C., and Fine, P.E.M. (1984) Assessment of the protective efficacy of vaccines against common diseases using case-control and cohort studies. Int. J. Epidemiol. 13(1):87-93. http://www.ncbi.nlm.nih.gov/pubmed/6698708. Accessed September 6, 2014.
[20] Vesikari, T., Prymula, R., Schuster, V., Tejedor, J.C., Cohen, R., Bouckenooghe, A., Damaso, S., and Han, H.H. (2012) Efficacy and Immunogenicity of Live-attenuated Human Rotavirus Vaccine in Breast-fed and Formula-fed European Infants. Pediatr. Infect. Dis. J. 31(5):509-13. http://www.ncbi.nlm.nih.gov/pubmed/22228235. Accessed September 6, 2014.
[21] De Serres, G., Boulianne, N., Meyer, F., and Ward B.J. (1995) Measles Vaccine Efficacy during an Outbreak in a Highly Vaccinated Population: Incremental Increase in Protection with Age at Vaccination up to 18 Months. Epidemiol. Infect. 115(2): 315-23. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2271410. Accessed September 6, 2014.
|








